Aromatics from Furans
As steam cracker operators are capitalizing on the shale gas revolution and inexpensive natural gas liquids are pushing naphtha feedstocks aside, the development of alternative routes for the production of aromatics from renewables, such as biomass, is receiving considerable attention.
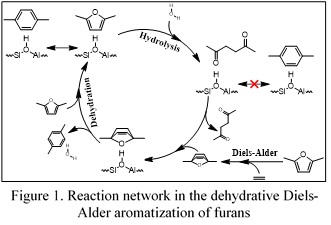
Of the possible routes garnering interest, CCEI scientists have proposed and been pursuing the dehydrative aromatization of the Diels-Alder product between bio-based furans with an appropriate dienophile; a tandem scheme where the cycloadduct obtained from the Diels-Alder is catalytically dehydrated to an aromatic. The strategy is showcased by the formation of p-xylene from 2,5-dimethylfuran and ethylene over the Brønsted-acidic catalysts H-Y and H-BEA, with yields as high as 90%. The same strategy has been used to convert methylfuran and ethylene to toluene over H-BEA; but methylfuran loss to side reactions (e.g., alkylation, hydrolysis and oligomerization) has an adverse effect on the selectivity (46%).
Lewis acid catalysis. In order to curtail undesired side reactions promoted by Brønsted acids, the CCEI researchers have turned their attention to Lewis-acidic zeotypes, specifically isomorphically substituted zeotypes with Lewis-acidic centers, such as Sn-BEA, Zr-BEA, Ti-BEA, Hf-BEA and Zn-BEA. This multi-institution effort led to the synthesis of numerous aromatics: methyl 4-(methoxymethyl)-benzoate from ethylene and methyl 5-(methoxymethyl)-furoate; dimethyl terephthalate from ethylene and the dimethyl ester of 2,5-furandicarboxylic acid; as well as p-xylene.
These efforts led to the remarkable finding that Lewis-acidic zeotypes are as effective as the Brønsted-acidic H-BEA in influencing the rate of the tandem scheme, and in particular the dehydration of oxanorbornene derivatives.
Catalysis of the Diels-Alder. The fleeting existence of the Diels-Alder cycloadduct has not allowed the CCEI engineers to decouple the two steps of the tandem scheme and thus elucidate whether the zeotypic Brønsted and Lewis acids have any effect on the rate of the Diels-Alder step itself.
With furans being among the less reactive dienes, accelerating the cycloaddition reaction will be a pivotal step in the success of producing aromatics by dehydrative aromatization of the Diels-Alder product of biomass-derived furans and appropriate dienophiles.
Brønsted and Lewis acids are known to accelerate a variety of Diels-Alder reactions. The mechanism by which they influence the rate of the DA reaction can readily be explained in terms of the famous Fukui theory of frontier molecular orbitals. In the so-named normal electron demand mode of the reaction, protonation of the dienophile or complexation with the Lewis acid lowers the energy of its lowest occupied molecular orbital and closes the gap to the highest occupied molecular orbital of the diene, increasing the interaction between the two orbitals and thus the rate of the reaction.
Calculations by CCEI scientists have, however, asserted that when ethylene is the dienophile, neither Brønsted nor Lewis acid zeotypes can influence the rate of the Diels-Alder step, because the furanic molecule binds more strongly to the active site of the catalysts.
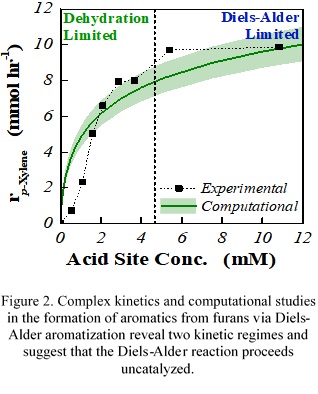
Breaking through. Recently, CCEI catalysis experts have achieved to synthesize methylbenzoate by Diels-Alder aromatization of furan and methylacrylate over the zeotypic Lewis acids Sn-BEA, Zr-BEA, and Hf-BEA. With the aid of computational chemistry, they were able to show that these Lewis acids are capable of accelerating the Diels-Alder reaction heterogeneously.
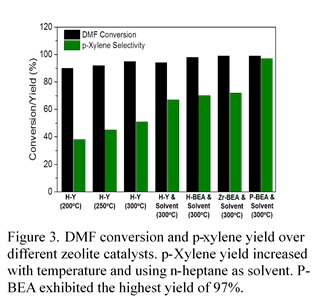
In order to curtail by-product formation, CCEI researchers have also been exploring phosphorous-containing siliceous zeolites. The have been able to synthesize zeolites with BEA (P-BEA) and MFI topology (P-SPP) and found that these catalysts are highly selective and stable for this reaction with an unprecedented p-xylene yield of 97%. The superior properties of phosphorous-containing siliceous zeolite catalysts are attributed to the nature of the acid sites which exclusively catalyze the dehydration of cycloadduct intermediate, and remarkably minimize the alkylation and oligomerization reactions.
Impact. Given the importance of the Diels-Alder cycloaddition as a tool in synthetic organic chemistry and in particular its significance for the development of a technology for the sustainable conversion of furans to aromatics, these are significant steps toward overcoming a number of undesirable or inconvenient features of traditional Lewis acids, such as sensitivity to water, or strong binding between the Lewis acid and the reactant or the product, which can slow down exchange and catalyst turnover.
