Searching the Adsorbate Chemical Space Reveals Reaction Feasibility and Discovers Suitable Catalysts
CCEI findings accelerate research in biomass conversion and waste recycling
Mechanistic understanding of large molecule conversion and the discovery of suitable heterogeneous catalysts have been lagging due to the combinatorial inventory of intermediates and the inability of humans to enumerate all structures. While the advancement of simulation methods has enabled in silico design of catalysts for electrochemical conversion of small molecules, this powerful technique has not been applicable for larger molecule conversion on metal catalysts as the number of intermediates and configurations explodes exponentially. The main problem has been automatically creating adsorption configurations, which describe the connectivity pattern between the adsorbates and the surface.
Researchers in the Catalysis Center for Energy Innovation (CCEI) at the University of Delaware have introduced an automated framework to predict stable configurations on transition metal surfaces and have demonstrated its validity for adsorbates with up to 6 carbon and oxygen atoms on 11 metals, enabling the exploration of ~108 potential configurations.
The team’s work was published in Nature Communcations on April 26, 2022.
An advanced graph theory method automatically builds a big adsorbate database
A newly developed algorithm systematically creates all possible adsorbate configurations by using carefully designed graph transformation rules, and the configuration stability is assessed by force-field optimization, multi-fidelity density functional theory calculations, and machine learning. The algorithm assesses X1 – X6 (X = C, O) adsorbates on close-packed surfaces of 11 metals. The density functional theory (DFT) identifies 4,979 stable adsorbates for X1 – X3, and machine learning identifies ~105 stable adsorbates for X3 – X6.
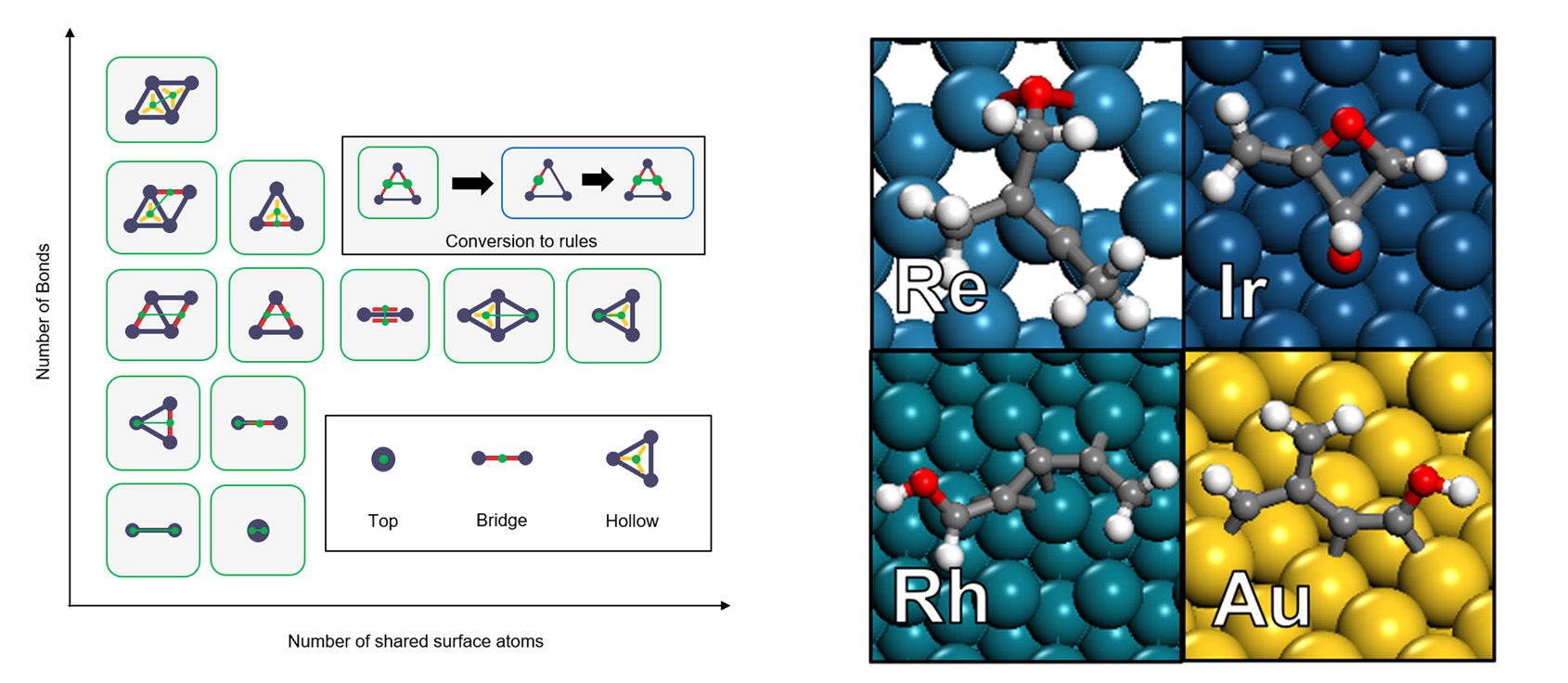
Systematic graph rule creation
Structures of large adsorbates
Analysis of the adsorbate space reveals that Ag and Cu are selective in the formation of epoxides by stabilizing oxocyclic intermediates
The principal component analysis of the preliminary database developed using the algorithm reveals an interesting insight. Ag and Cu can adsorb oxocyclic intermediates while nine other novel catalysts could not. Interestingly, Ag and Cu have been used as selective catalysts for the formation of epoxide. This indicates that new selective catalysts can be discovered by identifying those that selectively adsorb key intermediates.
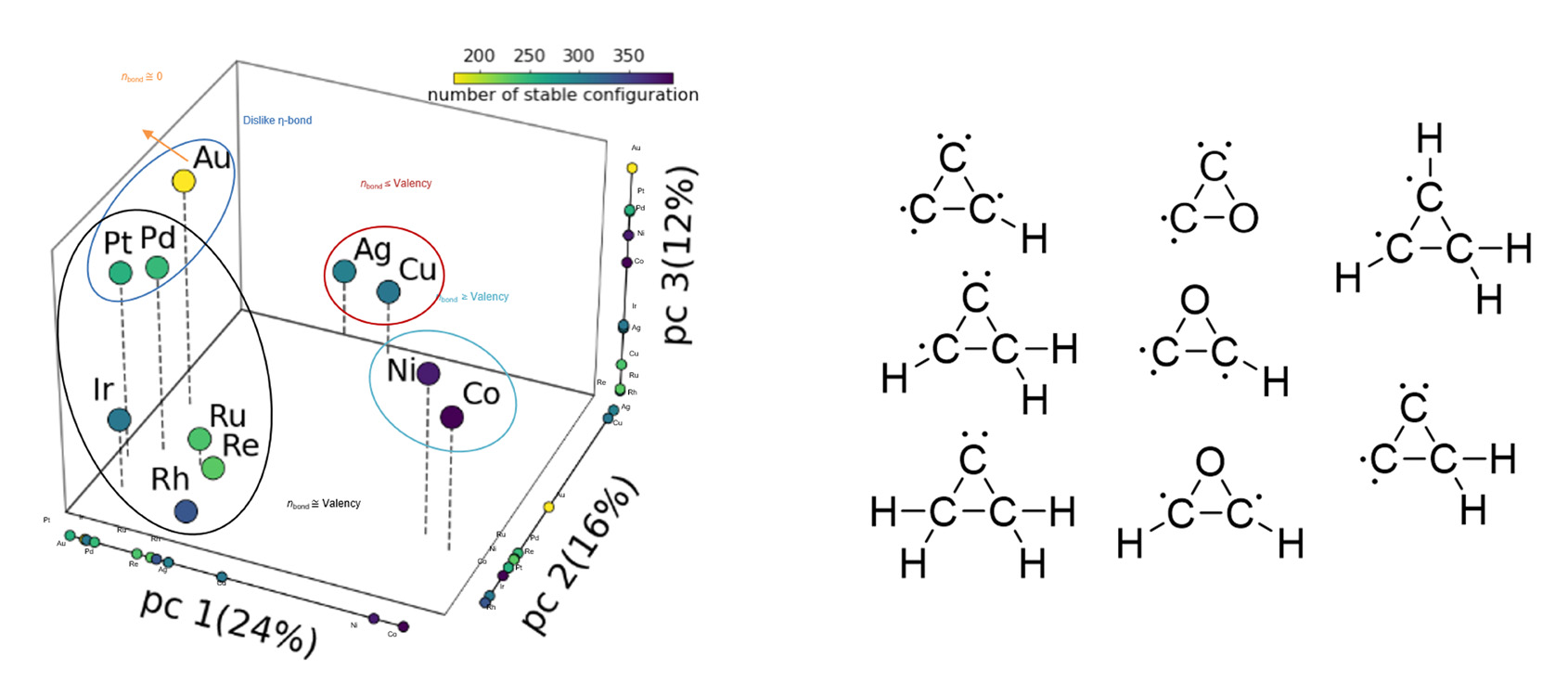
Adsorbate Chemical Space
Stable on Ag, Cu
This work paves the foundation toward large molecule conversion and the extensive library created through this work can be leveraged to pre-screen catalysts for all commonly metal-catalyzed chemistries. Establishing a framework for modeling large molecules significantly accelerates mechanistic and discovery studies, for example, in renewable energy, such as biomass pyrolysis and gasification, biomass upgrade via hydrogenation and hydrodeoxygenation, and hydrogen production via biomass reforming, and recycling of plastics. In fact, the creation of a larger database is underway at CCEI to accelerate the research for the large molecule conversion which includes biomass conversion and waste recycling.
The team included researchers from the University of Delaware, Korea Institute of Energy Technology (KENTECH), and Korea Advanced Institute of Science and Technology (KAIST).
The Catalysis Center for Energy Innovation (CCEI), an Energy Frontier Research Center (ERFC), was founded in 2009 and is funded by the Department of Energy (DOE).
